Document Management
Your Documents, Your Rules, Our System
A Enterprise Document Management System is designed to efficiently organize, store, manage, and control documents and records associated with quality management process within an organization. This system offers a centralized and structured approach to handling a wide range of document types, including policies, procedures, work instructions, forms, templates, reports, and other quality-related content.
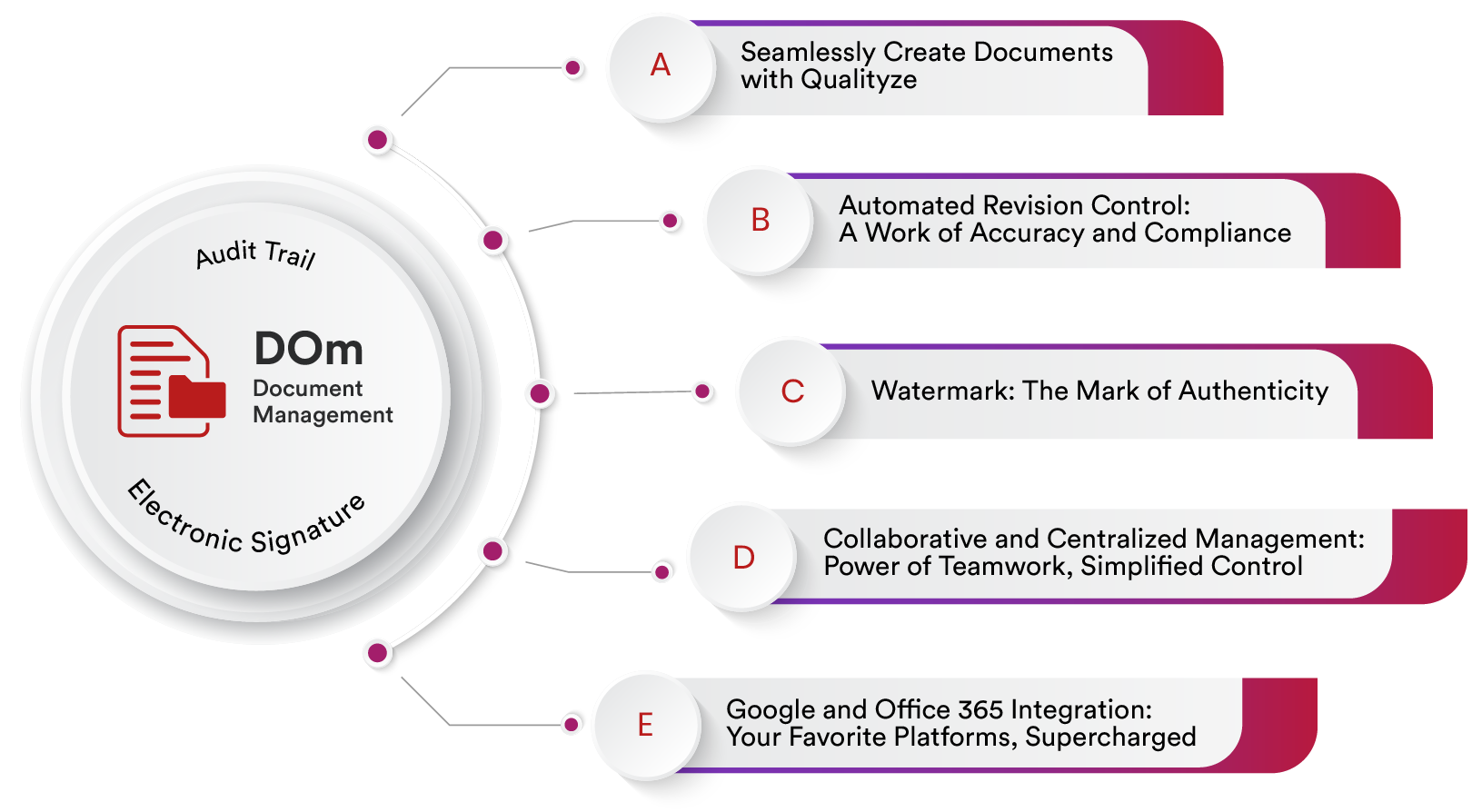
Enterprise Document Management System
Qualityze Document Management solution simplifies the management of the growing volume of documents by providing a robust framework and convenient sharing and tracking capabilities. With Qualityze, you can streamline your document process and empower your team to achieve document control excellence. When you use Qualityze Document Management solution, you can ensure that your organization stays on top of its documentation needs, reducing the risk of errors and non-compliance.

Seamlessly Create Documents with Qualityze
Creating documents shouldn't be a complex task. Qualityze user-friendly interface ensures that enterprise document management creation is a straightforward and intuitive process. Even if you're not a tech-savvy individual, you'll find yourself comfortably navigating the tools to generate various types of quality-related content. Whether it's policies, procedures, work instructions, templates, or any other document, our platform empowers you to craft them effortlessly.

Automated Revision Control: A Work of Accuracy and Compliance
Document revisions are an integral part of any workflow, but they can quickly become complex without proper management. Keeping track of document revisions manually can be arduous and to error. Qualityze offers an intelligent automated revision control system that streamlines the process, guaranteeing precision and compliance at every stage. Moreover, effortlessly identifying, retrieving, and comparing various document revisions makes the revision process seamless and efficient. This functionality also simplifies the demonstration of compliance during audits or inquiries, ensuring a smooth and hassle-free experience.

Watermark: The Mark of Authenticity
Watermarks have historically been used to signify authority or authenticity. Qualityze solution takes this concept to the digital realm. With Qualityze you can apply a unique watermark to each document, incorporating elements like company logos, identifiers, and printed timestamps. Every time a document is printed, Qualityze watermark feature leaves a traceable record. This record includes who printed the document and when it was printed. This transparency adds a layer of accountability, discouraging unauthorized printing and distribution. Additionally, you can also set a validity period for printed documents, which means that even if a document falls into the wrong hands, its validity is restricted to a certain timeframe, reducing the risk of misuse.

Collaborative and Centralized Management: Power of Teamwork, Simplified Control
Efficiency thrives in an environment of collaboration, but managing documents with multiple contributors can be challenging. Qualityze solution bridges this gap by providing collaborative and centralized document management. Qualityze allows multiple users to collaborate on document creation and revision in real time. Whether your team is in the same office or spread across the globe, everyone can work together seamlessly. With a centralized platform, Qualityze ensures that everyone is working on the same version of a document. No more confusion caused by multiple versions floating around.

Google and Office 365 Integration: Your Favorite Platforms, Supercharged
In today's digital landscape, productivity often hinges on the tools we use. Qualityze offers seamless integration with Google and Office 365, enhancing your document management experience. By leveraging the integration, you can harness the full power of Google and Office 365 while maintaining control and security through Qualityze. With this capability Qualityze allows you to author, manage, and collaborate on documents directly within these platforms. This integration eliminates unnecessary steps, saving you time and effort. This leads to smoother workflows and quicker results.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
Qualityze Document Management helps you save on the Cost of Quality. By streamlining and automating your document management processes, it reduces the time and effort spent on manual document handling. Efficient document control, versioning, and tracking ensure regulatory compliance, minimize errors, and improve productivity, resulting in significant cost savings.
Qualityze Document Management allows you to save on the Cost of Quality by:
- Streamlining document control, reducing manual efforts and administrative costs.
- Ensuring regulatory compliance and minimizing errors through efficient versioning and tracking.
- Enhancing collaboration and accountability through streamlined workflows and automated notifications.
- Improving productivity and efficiency, reducing costs associated with document-related delays and errors.
- Providing real-time data tracking and insights to make informed decisions and optimize resource allocation.
With Qualityze Document Management, you can optimize your document processes, reduce costs, and improve overall quality in a cost-effective manner.
Recognitions



Document Management Benefits to Various Operational Functions
Every business function has unique expectations from a solution, find out what Qualityze can deliver from your function's perspective.
Quality Assurance
Document management plays a vital role in quality assurance by ensuring efficient control, organization, and accessibility of critical documents. It streamlines processes, enables version control, and facilitates compliance.
Risk Management
Document management provides a systematic approach to document storage, version control, and access permissions. It ensures compliance, facilitates timely audits, and enables quick retrieval of critical information, reducing risks and enhancing decision-making processes.
Process Improvement
Document Management facilitates process improvement by streamlining document handling, reducing errors, and enhancing collaboration. It ensures easy access, version control, and efficient workflows, leading to increased productivity and compliance.
Supply Chain Management
Document management streamlines supply chain management by ensuring seamless coordination, efficient communication, accurate record-keeping, and informed decision-making. It enables real-time access to essential documents, enhances collaboration, and simplifies compliance.
Production Management
Document Management ensures efficient organization, retrieval, tracking and version control of crucial production documents for enhanced productivity and operational excellence. It streamlines workflows, improves collaboration, and maintains regulatory compliance.
Compliance and Regulatory Affairs
Document Management ensures efficient organization, storage, and retrieval of critical documents, facilitating compliance with regulations. Streamline audits, track document versions, and maintain a secure repository, empowering your organization to meet regulatory requirements seamlessly.
Experience Qualityze Difference in managing Quality
We empower organizations to uphold exceptional quality standards while providing the following advantages:
Configurable Cloud-Based Platform
Our system is developed on the world's foremost cloud-based platform, Salesforce.com, which allows businesses to effortlessly personalize it to their exact requirements. It is an adaptable and scalable system that expands in tandem with the growth of your organization. Its wide-ranging configurability broadens the functional scope of an EQMS to an infinite array of creative applications, making it an extremely valuable solution for your organization.
Compliance Ready
Our system adheres to compliance standards for every industry and follows the relevant regulations set by regulatory committees, such as the Food and Drug Administration (FDA) - 21 CFR Part 820, International Organization for Standardization (ISO) - ISO 9001, ISO 13485, Pharmaceuticals and Medical Devices Agency (PMDA), Therapeutic Goods Administration (TGA), China Food and Drug Administration (CFDA), and others.
User-Friendly Interface
Our system design makes it easy for users to accomplish their goals and complete tasks, while minimizing errors and confusion. It considers the needs of various user groups, and provides clear and helpful feedback throughout the user journey. Qualityze strives to create an emotional connection with the user, resulting in increased satisfaction, loyalty, and a positive brand image.
The Start of Something Amazing.
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
- Have better document control
- Maintain security and safety of the documents
- Eliminate errors and have better retention
- Improve decision-making and insigh
Why Document Management Is Important?
Document Management is the foundation for any modern quality management system. It includes the creation, storage, review, and modification of business-critical documents in a systematic and streamlined manner. Such a system enables you to:
How Qualityze transforms your Organization’s Document Management Process into A Winning Process?
Qualityze transforms your organization's document management process into a winning process by offering a robust and reliable software solution. Our cloud-based system eliminates the hassles of traditional document management, including storage limitations. You can easily upgrade your storage as required.
With our best-in-class document management system, you can streamline your entire document lifecycle, from creation to approval, archival to deletion, in a structured manner. Our solution enables you to create a centralized repository for your important documents, ensuring that only authorized individuals can access and assess them. As an administrator, you have control over setting approval dates, publishing dates, deletion schedules, archival periods, and follow-ups well in advance.
By utilizing Qualityze Document Control Management System, your documents are securely stored in a safe vault accessible only to authorized personnel who can modify or delete them. The built-in scheduler simplifies document-related tasks, even when you're not physically present, by sending reminders on specified dates to designate individuals. This ensures timely information delivery to end-users, promoting efficiency and productivity within your organization.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
Company
© 2025 Qualityze | All rights reserved. | Privacy Policy

















